Introducing Galleri®
Screen for many of the deadliest cancers through a single blood test1,2
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.

The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
How the Galleri test works
The Galleri test looks for a unique “fingerprint” of cancer by analyzing methylation patterns of cell-free DNA (cfDNA).1,5,6 If detected, the test predicts the most likely origin of the cancer signal, to help guide the diagnostic workup.1,7
The Galleri test is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer. The Galleri test identifies DNA in the bloodstream shed by cancer cells and does not predict future genetic risk for cancer. It should be used in addition to healthcare provider recommended screening tests.
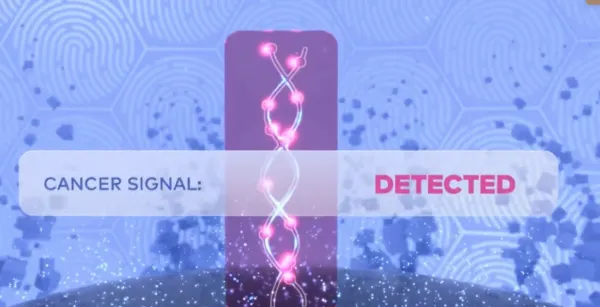
Now you can screen for multiple cancers with Galleri.
The Galleri test screens for a signal shared by 50+ types of cancer1:
In clinical studies, the following cancers were diagnosed after a Cancer Signal Detected result.1
Galleri is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer.
Supported by robust clinical data, Galleri sets the standard for multi-cancer early detection
20,000+ individuals participated in large clinical studies1,5

Hear from Galleri patients and providers
Galleri is revolutionizing cancer screening1
Get started
Complete this form and a GRAIL representative will reach out to you.
Not a healthcare provider? There are other options for contacting us here.
-
What describes you best?
-
What are you looking for?
-
Fill in the form
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Did you know?
You can order Galleri through Quest, Athenahealth, or TRICAREBy submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-77. DOI: https://doi.org/10.1016/j.annonc.2021.05.806.
- American Cancer Society. Lifetime Risk of Developing or Dying From Cancer. https://www.cancer.org/cancer/risk-prevention/understanding-cancer-risk/lifetime-probability-of-developing-or-dying-from-cancer.html.
- American Cancer Society. Cancer Facts & Figures 2022. Atlanta: American Cancer Society; 2022 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html. Data on file GA-2021-0065.
- US Preventive Services Task Force. A,B,C Grade Recommendations Cancer. https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results.
- Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016 Jul 8;35:347–76. doi: 10.1007/s10555-016-9629-x
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-59. doi: 10.1016/j.annonc.2020.02.011
- Schrag D, Beer TM, McDonnell CH L, et al. Blood-based tests for multi-cancer early detection (Pathfinder): a prospective cohort study. Lancet. 2023;42:1251-1260. doi:10.1016/S0140-6736(23)01700-2