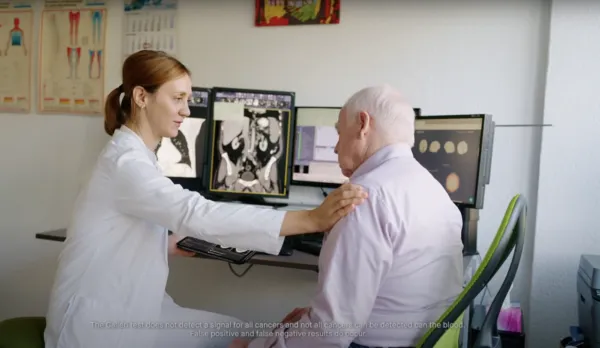
Screen for 50+ cancers with a single blood test
The Galleri® multi-cancer early detection test identifies DNA in the bloodstream shed by cancer cells1 and does not predict future genetic risk for cancer.
HSA/FSA eligible*
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
*Check with your plan to confirm eligibility
Now there’s a proactive tool to screen for cancer
The Galleri test can be taken annually as a simple blood test and screens for a “fingerprint” of many of the deadliest cancers before they become symptomatic, including those with no recommended screening tests today.1,2,3
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.



Healthcare Providers
Healthcare Providers
Healthcare Providers
I am a healthcare provider interested in offering the Galleri test to my patients

Employers
Employers
Employers
I represent an employer interested in offering the Galleri test to my organization’s employees

Health Systems
Health Systems
Health Systems
I represent a health system interested in making the Galleri test available to my patient population
Have more questions?
Please answer the following questions to fill in the appropriate form.
You can also contact us at (833) 694‑2553 for additional help.
-
What describes you best?
-
What are you looking for?
-
Fill in the form
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Billing Support
By submitting this form, you agree to GRAIL’s use of this information to contact you. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Did you know?
You can order Galleri through Quest, Athenahealth, or TRICAREBy submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Do you represent a first responder or firefighter organization? Use this form instead.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Galleri helps you to go further with cancer screening
The Galleri test gives you more control when it comes to cancer.
In a clinical study, adding the Galleri test to recommended single-cancer screenings approximately doubled the number of cancers detected.1,3,6

Learn how screening with Galleri has made a difference for these patients
We collaborated with leading cancer institutes to study the Galleri test:
Supported by robust clinical data, the Galleri test has been studied in multiple clinical trials with over 20,000 participants.3,6

Save $125 off the Galleri® test now.
The discount only applies to full-priced, self-pay tests priced at $949.* Most health insurance plans do not cover the Galleri test.
* Limited time offer valid only for Galleri test orders placed February 5, 2026, through March 19, 2026, 11:59 pm PST. Valid only for Galleri test orders requested online and approved by the GRAIL-contracted independent telemedicine provider. Offer does not confirm your eligibility for the Galleri test. Valid only for one-time use on domestic US Galleri test orders only. Offer valid only for a discount off the Galleri test list price ($949). Offer cannot be: (1) combined or used with any other discount, promotion, special pricing, or payment plan; (2) used to request a credit on a test order placed prior to the offer period; or (3) posted online or on any other publicly available forum. GRAIL reserves the right to change the terms and conditions, substitute an offer of equal or greater value, and end the offer at any time without notice. For Galleri test orders requested online, you must create an account to redeem this offer and enter the code at the “Introduction” stage, where prompted.

Resources
Sign up for more information about Galleri
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes
‡ Nabavizadeh N, et al. Safety and Performance of a Multi-Cancer Early Detection (MCED) Test in an Intended-Use Population: Initial Results from the Registrational PATHFINDER 2 Study. Proffered Presentation Presented at: European Society for Medical Oncology (ESMO) Annual Meeting; October 17-21, 2025; Berlin, Germany.
- US Preventive Services Task Force. A,B,C grade recommendations, cancer, screenings. [cited 2023 Oct 23]. https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021 Sep;32(9):1167-77. doi: 10.1016/j.annonc.2021.05.806
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020 Mar 30;31(6):745-59. doi: 10.1016/j.annonc.2020.02.011
- M. Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016 Jul 8;35:347–76. doi: 10.1007/s10555-016-9629-x
- Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER Research Limited-Field Data, 21 Regs, 2020 Nov Sub (2000-2018) - Linked To County Attributes - Time Dependent (1990-2018) Income/Rurality, 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released 2021 Apr, based on the 2020 Nov submission. [Risk Factor Data on file: American Cancer Society Cancer Prevention Studies II/III.]
- American Association for Cancer Research. Cancer progress report 2023. https://cancerprogressreport.aacr.org/progress/
- Schrag D, Beer TM, McDonnell CH, et al. Blood-based tests for multi-cancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402:1251-1260. doi:10.1016/S0140-6736(23)01700-2
- American Cancer Society Cancer Facts and Figures 2023. Available at: http://www.cancer.org/content/.... GRAIL, Inc. Data on file GA-2021-0065