Get ahead of cancer before symptoms appear
Cancer often hides, with symptoms not appearing until later stages. The earlier that cancer is diagnosed, the greater the chance of successful treatment and survival.1,2,3

Only 5 types of cancer have recommended screening tests4
Today, doctors test individually for 5 specific cancers — colorectal, lung (for those at risk), breast, cervical, and prostate.
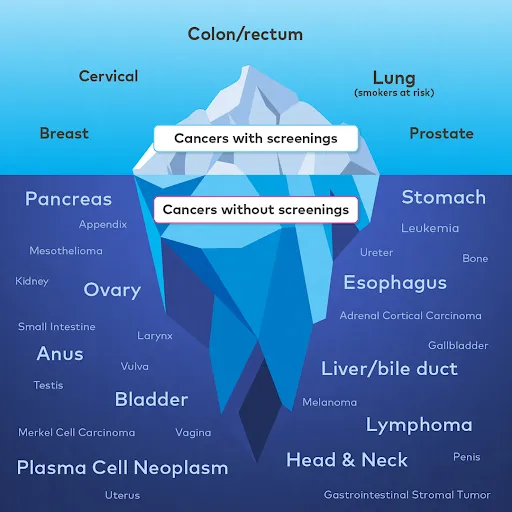
While these single-cancer screenings play an important role in detecting 5 specific cancers today, we can do more.
When cancer is diagnosed early,* before it has time to spread, the overall 5-year cancer-specific survival rate is 4 times higher than when cancer is diagnosed late.8*
*“Early/Localized” includes invasive localized tumors that have not spread beyond the organ of origin; “Late/Metastasized” includes invasive cancers that have metastasized beyond the organ of origin to other parts of the body.
More than 1 in 3 people will develop cancer in their lifetime2
Age is the biggest risk factor for cancer. People over 50 have a 13 times higher risk for cancer than those under 50. Cancer risk increases for everyone as they age, regardless of family history3 — only 5% to 10% of cancers are inherited.3
Certain factors, in addition to age 50+, further increase cancer risk4:
- Current or previous smokers
- Diabetes
- Obesity (BMI > 30)

The Galleri test allows you to go further with cancer screening
Sign up for more information about Galleri
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes
American Cancer Society. The cancer atlas. [Internet] Early detection. https://canceratlas.cancer.org/taking-action/early-detection/
NIH/National Cancer Institute. Detect Cancers Early - National Cancer Plan. https://nationalcancerplan.can...
Crosby D, Bhatia S, Brindle KM, et al. Early detection of cancer. Science. 2022 Mar 18;375(6586):eaay9040. doi: 10.1126/science.aay9040.
US Preventive Services Task Force. A,B,C grade recommendations, cancer, screenings. [cited 2023 Oct 23]. https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results
SEER Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2017 Sub. Includes persons aged 50+ diagnosed 2006-2015. Data on file GA-2021-0065
American Cancer Society. Lifetime risk of developing or dying from cancer. [cited 2023 Oct 11]. https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dying-from-cancer.html
NIH, National Cancer Institute. Genetic testing for inherited cancer susceptibility syndromes. [cited 2023 Mar 3]. https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
American Association for Cancer Research. Cancer progress report 2023. https://cancerprogressreport.aacr.org/progress/