Cancer needs a quick response time too
About 70% of cancer deaths are caused by cancers with no recommended screening.1,2*
It’s time for a change: Get multi-cancer early detection (MCED) screening with the Galleri® test.
*Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on the estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.

*Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on the estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.
“The bottom line for me was that screening with the Galleri test meant we could cast a wider net by adding it to single cancer screening tests … an incredible advancement for firefighter health.”

30,000+ first responders3 tested in 160+ US departments4





Screen for 50+ cancer types5
Galleri is a proactive blood test that screens for many of the deadliest cancers, including 12 cancers* responsible for about 2/3 of cancer deaths.1,5 If a cancer signal is detected, the Galleri test predicts the cancer signal origin with 93.4% accuracy.6 The test can be done annually in addition to recommended screenings (i.e., lung, for those at risk, colorectal, breast, prostate, and cervical).1,2,5
Reach out to the Galleri team to learn more.
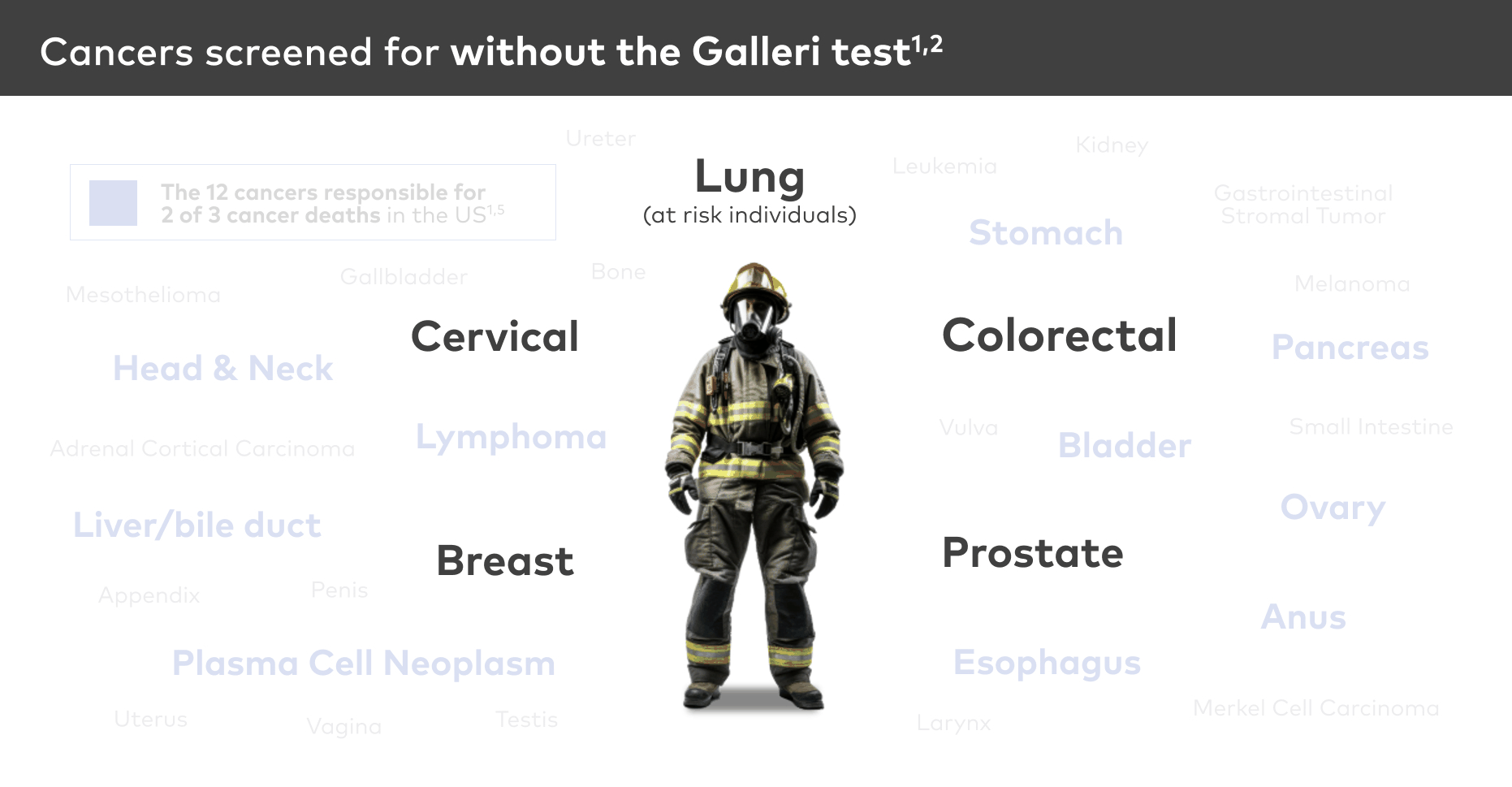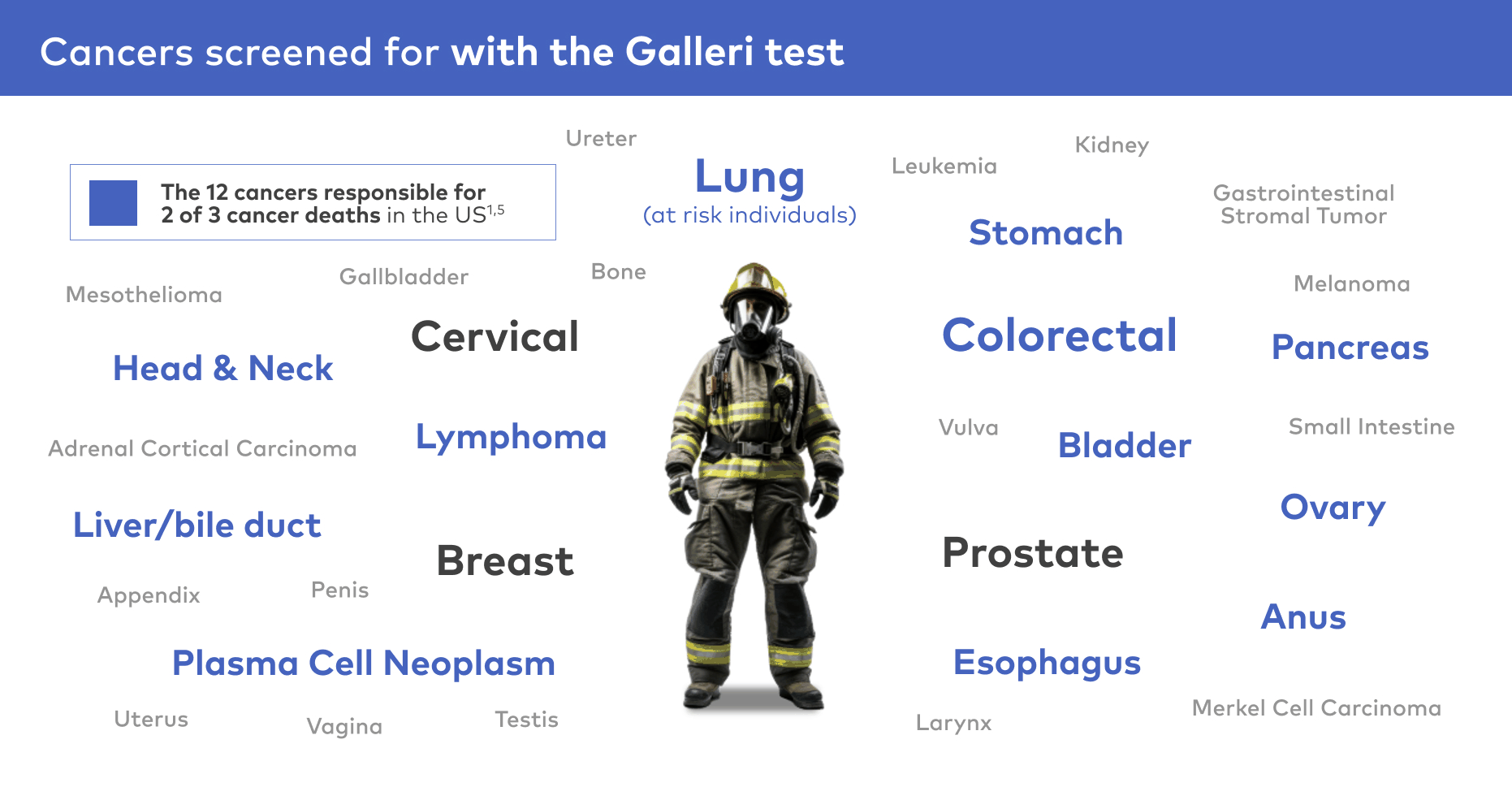
The Galleri test detects a cancer signal for 50+ cancer types, including those shown above.5 Please see test performance for test sensitivity details.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test identifies DNA in the bloodstream shed by cancer cells and does not predict future genetic risk for cancer. Galleri is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer. The Galleri test should be used in addition to healthcare provider recommended screening tests. The Galleri test is available by prescription only.
*Anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, and stomach.
Validated through clinical studies with over 20 leading institutions, including:5,10




How the Galleri test works
Using next-generation DNA sequencing, machine learning, and AI, the Galleri test can detect a cancer signal in the blood and predict the tissue or organ associated with the cancer signal.5
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test identifies DNA in the bloodstream shed by cancer cells and does not predict future genetic risk for cancer.
"What this test provided our firefighters and our department was priceless.”

The Galleri test is part of a comprehensive experience
GRAIL helps fire and police departments and other first responder organizations develop a robust engagement strategy to educate personnel (firefighters, police, EMS, and EMT teams) and raise awareness about the Galleri test.
There are 3 key ways to make the Galleri test available to your personnel:
- Onsite blood draw event: Most first responders take the Galleri test through in-person blood draw events, typically at their station.
- Telemedicine: Participants can request the Galleri test through a telemedicine provider and get their blood drawn through one of GRAIL's lab partners, including Quest Diagnostics, or at-home phlebotomy at no additional cost.
- Local health systems and PCPs: Local providers can order the Galleri test for first responders.
The Galleri test is available by prescription only. An independent provider will review a participant’s test request and determine if the test is right for them.
The test result report is delivered to the ordering provider and to the patient via email within 2-3 weeks after the sample is received at the GRAIL lab. If a Cancer Signal Detected result is received, the results are also communicated via a phone call or video consultation with the ordering provider. If the patient has an existing PCP, the results can be sent to the PCP if requested by the patient.
All individuals who receive a Cancer Signal Detected result are contacted by the ordering physician. Then, they receive a follow-up call from a patient advocate who can provide 360-degree case management, help coordinate the next steps, and even assist in finding an in-network primary care physician (PCP).
GRAIL also helps patients' providers navigate the next steps following a Cancer Signal Detected result. Resources include the Positive Result Resource Center, support from a Medical Science Liaison, and peer-to-peer consultation with an independent specialist and the Early Cancer Detection Board.
“An impressive 95% of our department participated.”
(In a Galleri on-site blood draw event)

Why first responders chose the Galleri test
"Our experience with GRAIL and the Galleri test has been
nothing short of amazing."

Get started
By submitting this form, you agree to GRAIL’s use of this information to contact you, including for marketing purposes. Please do not include any sensitive or confidential information, including health information. For more information, please refer to our privacy notice.
Frequently asked questions
The Galleri test screens for multiple cancers and can be taken annually as a simple blood draw. The test can screen for many aggressive cancers before symptoms appear.10
In a clinical study, the Galleri test detected a signal shared by more than 50 types of cancer.5 See the list of cancer types the Galleri test detected.
Learn more — watch the short video: What is Galleri?
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.
Cancers growing in the body shed DNA into the bloodstream. Although there are many types of cancer, the DNA fragments act like a unique fingerprint of cancer.5,11,12 The Galleri test can screen for many aggressive cancers before they become symptomatic,10 including those with no recommended screening tests.1,2,5 With this unique “fingerprint” of cancer, the Galleri test helps to provide direction to healthcare providers on the cancer’s origin and the next steps in diagnosis 6,10
Watch the short video How Does the Galleri Test Work? to learn more.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test identifies DNA in the bloodstream shed by cancer cells and does not predict future genetic risk for cancer.
First responders may request the Galleri test at a reduced cost. “First responders” include firefighters, police officers, and emergency medical service providers (whether active, volunteer, or retired) and spouses/dependents.
The Galleri test is available by prescription only. An independent provider will review a participant’s test request and determine if the test is right for them. The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. Use of the Galleri test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
The Galleri test should be used in addition to healthcare provider recommended screening tests.
No. A genetic or hereditary risk assessment is a one-time-only measurement and assesses future risk of developing cancer.13 The Galleri test is a point-in-time test that identifies DNA in the bloodstream shed by active cancer cells.5
In a clinical study, the Galleri test detected a signal shared by more than 50 types of cancer — including some fast-spreading and aggressive cancers responsible for approximately two-thirds of cancer deaths.1,5 Galleri is a cancer screening test, meaning it looks for cancer before symptoms appear.10
See the list of cancer types study participants had when a cancer signal was detected.11
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
Nearly 99% of people (ages 50-79) who take the Galleri test will receive a No Cancer Signal Detected result.10 In other words, approximately 1% are expected to receive a Cancer Signal Detected result. After diagnostic evaluation, around 62% of people with a Cancer Signal Detected result are expected to have a confirmed cancer diagnosis.9*
Some of the ways we measure test accuracy are with positive predictive value (PPV) and a false positive rate. A PPV is the probability that a person with a Cancer Signal Detected test result has cancer (i.e., 62%). The false positive rate was 0.4% for participants without cancer.9*
*Based on the first ~25,000 participants with 1 year of follow-up.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.
The Galleri test is commercially available throughout the United States and in select geographic locations through distributors.
There are three key ways that departments and unions make the Galleri test available to their personnel:
- Onsite blood draw event: Most first responders take the Galleri test through in-person blood draw events, typically at their station. We aim to make the testing process straightforward and convenient. We will work with you to determine the best location(s) and space requirements.
- Telemedicine: Participants can request the Galleri test through a telemedicine provider and get their blood drawn through one of GRAIL's lab partners, including Quest Diagnostics. At-home phlebotomy services are also available at no additional cost.
- Local health systems and PCPs: Local providers can order the Galleri test for first responders.
The Galleri test is available by prescription only. An independent provider will review a participant’s test request and determine if the test is right for them.
Watch the Clinical Laboratory Virtual Tour video to learn more about how samples are processed at the GRAIL lab.
No, fasting is not required for the Galleri test.
Typically, a participant can expect to receive their test result about 2-3 weeks after their sample arrives at the GRAIL lab. In certain cases, results may take up to 4 weeks.
It is important to understand that the timing of a test result does not indicate or predict the outcome of the test, nor does it impact the accuracy of the final result.
The Galleri test detected DNA methylation patterns often associated with cancer in the blood sample. About 1 out of every 100 tests has a Cancer Signal Detected result.10
This result will also include a prediction of the tissue type or organ associated with the cancer signal, called a Cancer Signal Origin (CSO). The CSO helps the participant’s doctor determine the next steps for diagnosis.5
A healthcare provider should interpret the results. The test result is not a cancer diagnosis and requires follow-up diagnostic testing, which may include lab work or imaging ordered by the participant’s healthcare provider to confirm cancer. GRAIL also offers patients and providers additional support and resources if needed to help guide the next steps.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
The Galleri test did not detect DNA methylation patterns associated with cancer in the blood sample. Nearly 99% of people who use the Galleri test will receive a No Cancer Signal Detected result.10
This result does not completely rule out the possibility of cancer, and the participant should continue with other cancer screenings their provider recommends. While the Galleri test is a powerful tool, it cannot detect a signal for all cancers and not all cancers can be detected in the blood. Some cancers shed little or no DNA into the bloodstream, which makes them unlikely to be detected through a blood test (e.g., brain, skin, and early breast and prostate cancers).14 False positive and false negative results do occur. The Galleri test identifies DNA in the bloodstream shed by cancer cells and does not predict future genetic risk for cancer.
The Galleri test can be taken as an annual blood test. Adding the Galleri test to yearly wellness visits can improve the chance of finding a cancer signal early, when more treatment options may be available.15 Participants can ask their healthcare provider when it is best to test again.
The Galleri test is intended to be used in addition to — and not replace — other cancer screening tests healthcare providers recommend. Single-cancer screening tests are recommended because they have been proven to save lives by detecting cancer early. Adding the Galleri test to annual wellness visits can improve the chance of finding a cancer signal early, when more treatment options may be available.15
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood.
A Cancer Signal Detected test result is not a cancer diagnosis and requires follow-up diagnostic testing to confirm cancer. Follow-up diagnostics may include lab work or imaging ordered by a healthcare provider to confirm cancer. GRAIL also offers patients and providers additional support and resources if needed to help guide the next steps.
Cancer can be unpredictable. Aggressive cancers can develop and progress quickly, sometimes in less than a year.15,16 The Galleri test can be taken annually. GRAIL can schedule annual onsite draw events, or participants can add the Galleri test to annual wellness visits. Participants can ask their healthcare provider when to test again.
Your department/union will not receive individual results. GRAIL only shares de-identified and aggregated information across all participants (e.g. how many tests have been requested) with the department/union.
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes
- American Cancer Society. Cancer facts & figures 2025. [GRAIL, Inc. Data on file: GA-2021-0065].
- US Preventive Services Task Force. A,B,C grade recommendations, cancer, screening [cited 2025 Mar 18].
- GRAIL, Inc. Number of First Responders Tested [Data on File GA-2024-0231]
- GRAIL, Inc. Number of Customers [Data on File GA-2024-0230].
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-77. doi: 10.1016/j.annonc.2021.05.806
- GRAIL, Inc. Enhanced Cancer Signal Origin prediction. [Data on file: VV-TMF-59592]
- GRAIL, Inc. Sponsor/Collaborator: GRAIL, Inc. ClinicalTrials.gov [accessed 2025 Jul 17]. https://clinicaltrials.gov/search?spons=GRAIL,%20Inc.&sort=StudyFirstPostDate&viewType=Table
- GRAIL, Inc. SYMPLIFY. ISRCTN ID: ISRCTN10226380 [accessed 2025 Jul 17]. https://www.isrctn.com/ISRCTN10226380
- Nabavizadeh N, et al. Safety and performance of a multi-cancer early detection (MCED) test in an intended-use population: initial results from the registrational PATHFINDER 2 study [presentation]. European Society for Medical Oncology (ESMO) Congress; 2025 Oct 17-21; Berlin. https://assets.grail.com/wp-content/uploads/2025/10/ESMO-2025_PF2-Initial-Results_Presentation_FINAL-CLEAN-10.16.2025.pdf
- Schrag D, Beer TM, McDonnell CH, et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402(10409):1251-60. doi: 10.1016/S0140-6736(23)01700-2
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-59. doi: 10.1016/j.annonc.2020.02.011
- Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-76. doi:10.1007/s10555-016-9629-x
- NIH, National Cancer Institute. Genetic testing for inherited cancer risk. [cited 2025 Jun 24]. https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
- Bredno J, Venn O, Chen X, Freese P, Ofman JJ. Circulating Tumor DNA Allele Fraction: A Candidate Biological Signal for Multicancer Early Detection Tests to Assess the Clinical Significance of Cancers. Am J Pathol. 2022 Oct;192(10):1368-1378. doi: 10.1016/j.ajpath.2022.07.007. Epub 2022 Aug 7. PMID: 35948080.
- Patel AV. Methylated DNA biomarkers and incident cancer in the American Cancer Society (ACS) Cancer Prevention Study-3 (CPS-3) cohort [presentation]. American Society of Clinical Oncology (ASCO) Annual Meeting; 2023 Jun 2-6; Chicago. https://assets.grail.com/wp-content/uploads/2023/06/Patel_ASCO-2023_ACS-CPS-3-Biobank_oral-presentation_FINAL.pdf
- Sasieni P, Clarke CA, Hubbell E. Impact of MCED screening interval on reduction in late-stage cancer diagnosis and mortality [poster]. European Society for Medical Oncology (ESMO) Virtual Congress; 2021 Sep 16-21. https://assets.grail.com/wp-content/uploads/2021/09/ESMO_Screening_Interval_Poster_G_Final_Submitted.pdf






