Unlock the potential of multi-cancer early detection
Galleri® is a multi-cancer early detection (MCED) test that identifies DNA from cancer cells in the bloodstream.1 It screens for a signal shared by many of the deadliest cancers that currently lack screening options.1,2 When a cancer signal is detected, Galleri predicts the most likely origin, helping guide the diagnostic workup.1,3
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur.
Cancer can affect anyone, but risk accelerates after age 50
Age is the biggest risk factor for cancer. Adults over the age of 50 are 13 times more likely to have cancer compared to people under the age of 50.4
Unfortunately, cancer can impact anyone. Approximately 90% of cancers are not hereditary.5

Currently, single-cancer screenings play an important role in detecting 5 specific cancers* (breast, cervical, colorectal, lung, and prostate).
These screenings have been shown to decrease cancer-specific mortality for the individual cancers that they detect. However, nearly 70% of cancer deaths are caused by cancers that don't have recommended screenings.2,6*
*Assumes screening is available for all prostate, breast, cervical, and colorectal cancer cases and 43% of lung cancer cases (based on estimated proportion of lung cancers that occur in screen-eligible individuals older than 40 years).
Adding the Galleri test to screen for more deadly cancers1,2
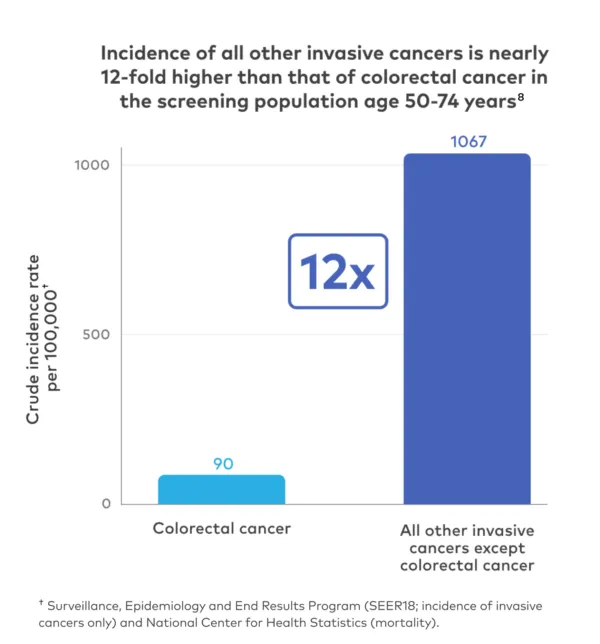
Clinical trials have shown that routine single-cancer screening tests save lives.7 However, an individual undergoing single-cancer screening, such as for colorectal cancer, is more likely to be diagnosed with a different cancer than the one being screened.8 The Galleri test goes further than routine cancer screenings to screen for more cancers, such as pancreatic and ovarian cancer.1,2*
Adding multi-cancer early detection gives healthcare providers a chance to screen for cancer while the patient is still asymptomatic, when cancer may be more easily treated and potentially curable.1,6
How the Galleri test works
Galleri leverages state-of-the-art DNA sequencing and pattern recognition technology to screen for the presence of a cancer signal and localize its most likely origin.1,9
DNA methylation is a process used by cells to regulate gene expression. Methyl groups are added to DNA to help turn genes on and off. In cancer, certain regions of DNA are often over- or under-methylated, disrupting the normal function of cells.10,11 The Galleri test is trained to recognize such cancer-specific methylation patterns.9
Galleri is a screening test and does not diagnose cancer. Diagnostic testing is needed to confirm cancer.
Screening for multiple cancers with Galleri
Cancer Signal Detection
The Galleri test analyzes cell-free DNA (cfDNA) fragments to detect abnormalities in methylation patterns that could indicate the presence of cancer. 1
The Galleri test has been trained on the largest known methylation database to identify patterns common to many cancers. It checks hundreds of thousands of methylation sites covering the most relevant genomic regions in DNA.9 The Galleri test can screen for a signal shared by more than 50 types of cancer.1

Cancer Signal Origin Prediction
If a cancer signal is detected, the Galleri test predicts the most likely tissue type or organ associated with the cancer signal to help guide the next steps to diagnosis. 1
The Galleri test uses pattern recognition to compare the patient’s cfDNA methylation pattern to the patterns of the possible Cancer Signal Origin (CSO) predictions. The test’s ability to predict the Cancer Signal Origin can help healthcare providers select appropriate diagnostic tests and procedures. 1,12

The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those age 50 or older. The test does not detect all cancers and should be used in addition to routine cancer screening tests recommended by a healthcare provider. The Galleri test is intended to detect cancer signals and predict where in the body the cancer signal is located. Use of the test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs, and symptoms. A test result of No Cancer Signal Detected does not rule out cancer. A test result of Cancer Signal Detected requires confirmatory diagnostic evaluation by medically established procedures (e.g., imaging) to confirm cancer.
If cancer is not confirmed with further testing, it could mean that cancer is not present or testing was insufficient to detect cancer, including due to the cancer being located in a different part of the body. False positive (a cancer signal detected when cancer is not present) and false negative (a cancer signal not detected when cancer is present) test results do occur. Rx only.
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167-77. doi: 10.1016/j.annonc.2021.05.806
- US Preventive Services Task Force. A,B,C grade recommendations, cancer, screening. [cited 2023 Oct 23]. https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results
- Schrag D, Beer TM, McDonnell CH, et al. Blood-based tests for multi-cancer early detection (PATHFINDER): a prospective cohort study. Lancet. 2023;402:1251-1260. doi: 10.1016/S0140-6736(23)01700-2
- Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER Research Limited-Field Data, 21 Regs, 2020 Nov Sub (2000-2018) - Linked To County Attributes - Time Dependent (1990-2018) Income/Rurality, 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released 2021 Apr, based on the 2020 Nov submission. [Risk factor data on file: American Cancer Society Cancer Prevention Studies II/III.]
- NIH, National Cancer Institute. Genetic testing for inherited cancer susceptibility syndromes [cited 2023 Mar 3]. https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
- American Cancer Society. Cancer facts & figures 2025. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html
- NIH, National Cancer Institute. Crunching numbers: what cancer screening statistics really tell us. [cited 2023 Mar 3]. https://www.cancer.gov/about-cancer/screening/research/what-screening-statistics-mean
- Clarke CA, Hubbell E, Ofman JJ. Multi-cancer early detection: a new paradigm for reducing cancer-specific and all-cause mortality. Cancer Cell. 2021 Apr 12;39(4):447-448. doi: 10.1016/jccell.2021.02.004
- Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-59. doi: 10.1016/j.annonc.2020.02.011
- Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016 Jul 8;35:347–76. doi: 10.1007/s10555-016-9629-x
- Nakajima T, Enomoto S, Ushijima T. DNA methylation: a marker for carcinogen exposure and cancer risk. Environ Health Prev Med. 2008;13:8-15. doi: 10.1007/s12199-007-0005-x
- Schrag D, McDonnall CH, Naduld L, et al. PATHFINDER: A Prospective Study of a Multi-Cancer Early Detection Blood Test. Presentation at European Society of Medical Oncology (ESMO) Congress September 9-13, 2022; Paris, France.